Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Momma, Yuichiro*; Sakairi, Masatoshi*; Ueno, Fumiyoshi; Otani, Kyohei
Zairyo To Kankyo, 71(5), p.133 - 137, 2022/05
The effect of the corrosion inhibitor on the corrosion of steel under a thin solution layer was investigated. As a result of forming a thin solution layer with a thickness of 1.0-0.2 mm on the specimen, adding a mixed solution of sodium molybdate and aluminum lactate as a corrosion inhibitor, and performing electrochemical measurement, the corrosion inhibitor suppresses the anodic reaction. And in the thin solution layer, it was suggested that the morphology of the protective layer structure by the corrosion inhibitor changed according to the amount of liquid as compared with the bulk immersion.

 generation; The Role of NO
generation; The Role of NO
 in the crevice corrosion repassivation of type 316L stainless steel
in the crevice corrosion repassivation of type 316L stainless steelAoyama, Takahito; Sugawara, Yu*; Muto, Izumi*; Hara, Nobuyoshi*
Journal of the Electrochemical Society, 166(10), p.C250 - C260, 2019/01
Times Cited Count:5 Percentile:17.1(Electrochemistry)The role of NO
 in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO
in the repassivation of crevice corrosion of Type 316L stainless steel was investigated. In crevice corrosion tests, the solution was changed from 1 M NaCl to NaCl-NaNO . NO
. NO
 led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm
led to complete repassivation. Repassivation of the crevice corrosion was found to take place in two steps. In the first step, the estimated current density inside the crevice gradually decreased from ca. 5 mA cm to ca. 5
to ca. 5  A cm
A cm . After that, the current density suddenly decreased to less than 0.1
. After that, the current density suddenly decreased to less than 0.1  A cm
A cm . From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
. From the potentiodynamic polarization in acidic solutions simulated inside the crevice (pH 0.2) and in situ observations of the crevice corrosion morphology, the first step was thought to be generated by the suppression of active dissolution by NO
 . It would appear that the generation of NH
. It would appear that the generation of NH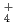 results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
results in a pH increase and the further suppression of active dissolution, and then repassivation occurs.
Maki, Akira; ; Taguchi, Katsuya; ; Shimizu, Ryo; Shoji, Kenji;
JNC TN8410 2001-012, 185 Pages, 2001/04
"The third technological meeting of Tokai Reprocessing plant (TRP)" was held in JNFL Rokkasyo site on March 14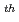 , 2001. The technical meetings have been held in the past two times. The first one was about the present status and future plan of the TRP and second one was about safety evaluation work on the TRP. At this time, the meeting focussed on the corrosion experrience, in-service inspection technology and future maintenance plan. The report contains the proceedings, transparancies and questionnaires of the meeting are contained.
, 2001. The technical meetings have been held in the past two times. The first one was about the present status and future plan of the TRP and second one was about safety evaluation work on the TRP. At this time, the meeting focussed on the corrosion experrience, in-service inspection technology and future maintenance plan. The report contains the proceedings, transparancies and questionnaires of the meeting are contained.
Taniguchi, Naoki; ; Kawasaki, Manabu*; Masugata, Tsuyoshi*
JNC TN8400 2001-001, 56 Pages, 2000/12
It is necessary to clear the effects of corrosion products on the corrosion life time of carbon steel overpack for geological isolation of high-level radioactive waste(HLW). Especially, it is important to understand the effects of magnetite because magnetite as a simulated corrosion product is reported to accelerate the corrosion rate of carbon steel. In this study, corrosion tests to reproduce the acceleration of corrosion due to magnetite was performed and the mechanism of the acceleration was investigated to evaluate the effects of magnetite as a corrosion product. Based on the results of experiments, following conclusions are obtained ; (1)Magnetite powder accelerates the corrosion rate of carbon steel. The main reaction of corrosion under the presence of magnetite is the reduction of Fe(III) in magnetite to Fe(II), but the reaction of hydrogen generation is also accelerated. The contribution of hydrogen generation reaction was estimated to be about 30% in the total corrosion reaction based on the experimental result of immersion test under the presence of magnetite. (2)Actual corrosion products containing magnetite generated by the corrosion of carbon steel protect the metal from the propagation of corrosion. The corrosion depth of carbon steel overpack due to magnetite was estimated to be about 1 mm based on the results of experiments. Even if the effect of magnetite is taken into the assessment of corrosion lifetime of overpack, total corrosion depth in 1000 years is estimated to be 33 mm, which is smaller than the corrosion allowance of 40 mm described in the second progress report on research and development for the geological disposal of HLM/ in Japan. It was concluded that the effect of magnetite on the corrosion life time of carbon steel overpack is negligible.
Akashi, Masatsune*; Fukaya, Yuichi*; Asano, Hidekazu*
JNC TJ8400 2000-014, 22 Pages, 2000/02
Difference of hydrogen generation phenomena on the surface of the Steels were not observed between carbon steel, atmospheric corrosion resisting steel and 5%-Ni steel. Rust layer was formed on these three-type of steels by steam oxidation method. And the chemical composition of the rust for the steels were basically two(2) layers structure for the previous two steels as hematite(Fe O
O ) based for the outer layer and magnetite(Fe
) based for the outer layer and magnetite(Fe O
O ) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe
) based for the inner layer. And for the last steel, it had three(3) layer in the rust as hematite(Fe O
O ) based for the outer layer, magnetite(Fe
) based for the outer layer, magnetite(Fe O
O ) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
) based for the intermediate layer and Ni based layer for the inner layer. These steels showed mostly same Tafel gradient in their cathodic polarization curves compare with that for no rust specimens. However, the exchange current density which reaction is assumed as a hydrogen generation reaction was largely increased. The cathodic reaction for each steels whose surface is covered by magnetite layer might be accelerated, then the corrosion rate was considered as accelerated, too.
*
PNC TJ9605 91-001, 28 Pages, 1990/10
The present report describes the results of studies performed sa a part of the results of "CORROSION TESTS OF CLADDING INNER SURFACE COATING" during a period of Feb. 20 - Mar.30, 1990. In the present study, corrosion tests have been carried out with CsOH to evaluate corrosion resistance of ferritic stainless steel and coated stainless steel. The following results were drawn from the present study (1)Corrosion tests of austenitic and ferritic stainless steel with ScOH were made at temperatures of 500-700 C. After corrosin tests, intergranular attack was found to occur in the austenitic steel, however, there was no intergranular attack in the ferritic steel. Ferritic steel appears to have corrosion resistance to liquid CsOH superior to austenitic steel. (2)Corrosion tests between Ni-Ti, Ti, Al coatings on stainless steel and CsOH were made at temperature of 500-700
C. After corrosin tests, intergranular attack was found to occur in the austenitic steel, however, there was no intergranular attack in the ferritic steel. Ferritic steel appears to have corrosion resistance to liquid CsOH superior to austenitic steel. (2)Corrosion tests between Ni-Ti, Ti, Al coatings on stainless steel and CsOH were made at temperature of 500-700 C. Ni-Ti and Al coated stainless steel showed no intergranular attack, though the coatings were locally detached from the stainless stell substrates. Intergranular attack was observed in the Ti coated stainless steel. Ni-Ti and Al coating seem to be useful for reduction of intergranular attack of stainless steel cladding.
C. Ni-Ti and Al coated stainless steel showed no intergranular attack, though the coatings were locally detached from the stainless stell substrates. Intergranular attack was observed in the Ti coated stainless steel. Ni-Ti and Al coating seem to be useful for reduction of intergranular attack of stainless steel cladding.
Otani, Kyohei; Ueno, Fumiyoshi; Kato, Chiaki
no journal, ,
In order to develop a novel corrosion inhibitor that is suitable for the 1F environment, it was focused on aluminum lactate, which had not been focused on as a corrosion inhibitor. It was found that aluminum lactate had a synergistic effect and high corrosion inhibition ability when mixed with sodium molybdate. As a result of surface XPS analysis, it was found that the corrosion of carbon steel was inhibited by the formation of aluminum and molybdenum oxide films on the carbon steel surface.